phosphorylation
Phosphorylation involves the addition of a phosphate group (PO4) to a protein or other molecule. Addition of PO4 renders the molecule more polar, and hence hydrophilic, and increases the Gibbs free energy potential of the recipient molecule. Phosphorylation is a very important reaction in cellular regulation, signaling and energy management. ATP synthase is the ultimate enzyme in oxidative phosporylation (table).
Specific protein kinases transfer a phosphate group from a donor such as ATP to amino acid acceptors in proteins, while protein phosphatases remove the phosphate groups that have been attached by protein kinases. Examples are receptor tyrosine kinases (RTKs), cytoplasmic protein tyrosine kinases (PTKs), and serine-threonine kinases.
Autophosphorylation is a property of most protein kinases, in which either serine and/or threonine or tyrosine serves as the phosphoacceptor for phosphorylation of self by a serine-threonine kinases or receptor tyrosine kinase. Several sites on the same kinase subunit are usually autophosphorylated in a manner that affects the functional properties of most protein kinases.[r]
Tables Phosphate-handling Enzymes Cell signaling RTKs
Not to be confused with phosphatases or kinases, phosphorylases are allosteric enzymes that catalyze the transfer of phosphate groups from an inorganic phosphate to an acceptor. Phosporylases are classified according to the acceptor molecule, and all phosphorylases share catalytic and structural properties. For example, glycogen phosphorylase attacks 1,4 glycosidic linkages in linear glycogens to generate glucose-1-phosphate, which is subsequently converted, by phosphoglucomutase (rev. isomutase) , into glucose-6-phosphate for glycolysis or the pentose-phosphate pathway. Debranching enzymes are required to attack 1,6 glycosidic branching points. Several enzymes possess separate phosphorylation sites for the activation or inhibition of functional regulation. For example, a subclass of serine/threonine protein kinases, CDKs can be either activated or deactivated depending upon the specific amino acid residue that is undergoing phosphorylated. Cyclin-dependent kinases (CDKs) play a role in regulation of transcription and in mRNA processing, and in regulation of the cell cycle. Studies with yeast and embryonic cells suggest that mitosis is triggered by the periodic activation of cdc2 kinase (cdk1). This enzyme is a member of the Ser/Thr protein kinase family, and is a catalytic subunit of the highly conserved protein kinase complex known as M-phase promoting factor (MPF), which is essential for G1/S and G2/M phase transitions of eukaryotic cell cycle. Mitotic cyclins form stable associations with cdk1 (cdc2), and function as regulatory subunits. The kinase activity of cdk1 is controlled by cyclin accumulation and destruction through the cell cycle. The phosphorylation and dephosphorylation of cdk1 play important regulatory roles in cell cycle control.
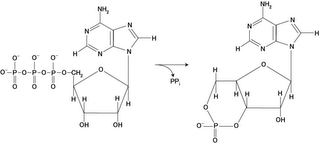
 Adenylyl (adenylate) cyclases are enzymes, which cross the membrane twelve times (right) and which convert ATP to the second-messenger cAMP (3',5' cyclic AMP) and pyrophosphate (below left). Likewise, guanylate cyclases convert GTP to the second messenger, cGMP. Adenylyl cyclases are coincidence detectors, meaning that they are only activated by several different signals occurring together – they are modulated by G-proteins, forskolin, Ca2+/calmodulin, and other class-specific substrates.
Adenylyl (adenylate) cyclases are enzymes, which cross the membrane twelve times (right) and which convert ATP to the second-messenger cAMP (3',5' cyclic AMP) and pyrophosphate (below left). Likewise, guanylate cyclases convert GTP to the second messenger, cGMP. Adenylyl cyclases are coincidence detectors, meaning that they are only activated by several different signals occurring together – they are modulated by G-proteins, forskolin, Ca2+/calmodulin, and other class-specific substrates.
Phospholipases are enzymes that hydrolyze specific ester bonds in phosphoglycerides or glycerophosphatidates, converting the phospholipids into fatty acids and other lipophilic substances. Phospholipases are involved in signaling cascades. Phospholipase A1 hydrolyzes the acyl group attached to the 1-position, while phospholipase A2 hydrolyzes the acyl group attached to the 2-position to form fatty acid and lysophospholipid products. Phospholipase A2 is responsible for the release of arachidonic acid from membranes (flow diagram PLA2 pathway). Arachidonic acid is a signalling molecule and is the precursor for eicosanoid signaling molecules, which include leukotrienes and prostaglandins. Some eicosanoids are synthesized from diacylglycerol, and are released from the lipid bilayer by phospholipase C.
Members of the five subtypes of phosphodiesterase enzymes degrade the cyclic nucleotide, second messengers, cAMP and cGDP by hydrolyzing phosphodiester bonds, so they are important in regulation of signal transduction. Phospodiesterase inhibitors, such as caffeine, aminophylline, theophylline and Viagra, prolong or amplify the physiological processes that are mediated by cAMP or cGDP. The phospodiester bond form the linkage between 3'-C of nucleotides and 5'-C of pentose sugars in the backbones of DNA & RNA, rendering phosphodiester bonds particularly important. 3'-phospodiesterase is important in repair of oxidative DNA damage.
Tables Phosphate-handling enzymes Cell signaling RTKs Second Messengers .
Examples of use of phosphorylation/dephosphorylation:
1. signaling networks and signal transduction (two-component system)
2. control of cell cycle
3. regulation of amino acids within Ser > Thr >>>>> Tyr
5. bacterial phosphorylation of the amino acids His and Asp in two-component signaling
6. p53 tumor suppressor gene
7. control of chemotaxis in prokaryotes (two-component system)
Examples of phosphorylation reaction
1. ADP + Pi →ATP synthase→ ATP : during oxidative phosporylation, substrate-level phosphorylation during glycolysis, and PSII light-reactions during noncyclic photophosphorylation (diagram Z-scheme)
2. NADH + Pi→NADPH in catabolic reactions such as photophosphorylation
Examples of dephosphorylation reaction
1. ATP - Pi → ADP in anabolic reactions
2. NADPH -Pi →NADH in anabolic reactions
Examples of signaling phosphorylation/dephosphorylation enzymes: two-component systems :
1. receptor tyrosine kinases (RTKs)
2. protein tyrosine phosphatases (PTPs) - transmembrane and intracellular - (CD45),
3. serine/threonine kinases (activins, inhibins, bone morphogenetic proteins (BMPs), MAP kinases, PKCs, TGF-beta receptors),
4. cAMP dependent protein kinase (Phospholipases (PLD, PLA2), Phosphatidylinositol-3-Kinase,
6. Phosphatases.
The spatio-temporal activation of protein kinases and phosphatases is an important factor in controlling where and when phosphorylation events occur. Anchoring proteins provide a molecular framework that orients these enzymes towards selected substrates. A-kinase anchoring proteins (AKAPs) are signal-organizing molecules that compartmentalize the cAMP dependent protein kinase, phosphodiesterases, and a variety of enzymes that are regulated by second messengers[s].
Phospholipases and phospholipids participate in transmission of ligand-receptor induced signals from the plasma membrane to intracellular proteins, primarily PKC, which is maximally active in the presence of calcium ion and diacylglycerol. PKC activity is mediated by receptors that are coupled to activation of phospholipase C-gamma (PLC-gamma), which contains SH2 domains that enable it to interact with tyrosine phosphorylated RTKs. PI-3K is tyrosine phosphorylated and activated by various RTKs and receptor-associated PTKs. PI-3K is activated by the PDGF, EGF, insulin, IGF-1, HGF and NGF receptors. The p85 subunit of PI-3K is activated by tyrosine phosphorylation, but only the 110 kDa subunit is enzymatically active.
Phospholipases D and A2 (PLD, PLA2) sustain the activation of PKC through their hydrolysis of membrane phosphatidylcholine (PC). Activation of PLC-gamma results in hydrolysis of membrane phosphatidylinositol bisphosphate (PIP2), which leads to an elevation of intracellular second messengers, diacylglycerol (DAG) and inositol trisphosphate (IP3), which interact with intracellular membrane receptors to effect release of stored calcium ions (PKC is maximally active in the presence of calcium ion and diacylglycerol).
Chemotaxis • hormones • neurotransmission • Nitric Oxide • neuronal interconnections • phosphotransfer-mediated signaling pathways • Protein Kinase Signaling Networks • protein tyrosine kinases, PTKs • receptor tyrosine kinases • Receptor Tyrosine Kinases (RTKs) • serine/threonine kinases • signaling gradients • signal transduction • two-component systems • animation MAPK signal transduction :
Signaling pathways:
Pathways ABC transporters : Phosphotransferase system (PTS) : Two-component system : MAPK signaling pathway : Wnt signaling pathway : Notch signaling pathway : Hedgehog signaling pathway : TGF-beta signaling pathway : VEGF signaling pathway : Jak-STAT signaling pathway : Calcium signaling pathway : Phosphatidylinositol signaling system : mTOR signaling pathway : Neuroactive ligand-receptor interaction : Cytokine-cytokine receptor interaction : ECM-receptor interaction : Cell adhesion molecules (CAMs) : Orthologies Transporters (+diseases) : Two-component system : Receptors and channels (+diseases) : Cytokines : Cell adhesion molecules (CAMs) : CAM ligands : CD molecules : GTP-binding proteins :
Specific protein kinases transfer a phosphate group from a donor such as ATP to amino acid acceptors in proteins, while protein phosphatases remove the phosphate groups that have been attached by protein kinases. Examples are receptor tyrosine kinases (RTKs), cytoplasmic protein tyrosine kinases (PTKs), and serine-threonine kinases.
Autophosphorylation is a property of most protein kinases, in which either serine and/or threonine or tyrosine serves as the phosphoacceptor for phosphorylation of self by a serine-threonine kinases or receptor tyrosine kinase. Several sites on the same kinase subunit are usually autophosphorylated in a manner that affects the functional properties of most protein kinases.[r]
Tables Phosphate-handling Enzymes Cell signaling RTKs
Not to be confused with phosphatases or kinases, phosphorylases are allosteric enzymes that catalyze the transfer of phosphate groups from an inorganic phosphate to an acceptor. Phosporylases are classified according to the acceptor molecule, and all phosphorylases share catalytic and structural properties. For example, glycogen phosphorylase attacks 1,4 glycosidic linkages in linear glycogens to generate glucose-1-phosphate, which is subsequently converted, by phosphoglucomutase (rev. isomutase) , into glucose-6-phosphate for glycolysis or the pentose-phosphate pathway. Debranching enzymes are required to attack 1,6 glycosidic branching points. Several enzymes possess separate phosphorylation sites for the activation or inhibition of functional regulation. For example, a subclass of serine/threonine protein kinases, CDKs can be either activated or deactivated depending upon the specific amino acid residue that is undergoing phosphorylated. Cyclin-dependent kinases (CDKs) play a role in regulation of transcription and in mRNA processing, and in regulation of the cell cycle. Studies with yeast and embryonic cells suggest that mitosis is triggered by the periodic activation of cdc2 kinase (cdk1). This enzyme is a member of the Ser/Thr protein kinase family, and is a catalytic subunit of the highly conserved protein kinase complex known as M-phase promoting factor (MPF), which is essential for G1/S and G2/M phase transitions of eukaryotic cell cycle. Mitotic cyclins form stable associations with cdk1 (cdc2), and function as regulatory subunits. The kinase activity of cdk1 is controlled by cyclin accumulation and destruction through the cell cycle. The phosphorylation and dephosphorylation of cdk1 play important regulatory roles in cell cycle control.
 Adenylyl (adenylate) cyclases are enzymes, which cross the membrane twelve times (right) and which convert ATP to the second-messenger cAMP (3',5' cyclic AMP) and pyrophosphate (below left). Likewise, guanylate cyclases convert GTP to the second messenger, cGMP. Adenylyl cyclases are coincidence detectors, meaning that they are only activated by several different signals occurring together – they are modulated by G-proteins, forskolin, Ca2+/calmodulin, and other class-specific substrates.
Adenylyl (adenylate) cyclases are enzymes, which cross the membrane twelve times (right) and which convert ATP to the second-messenger cAMP (3',5' cyclic AMP) and pyrophosphate (below left). Likewise, guanylate cyclases convert GTP to the second messenger, cGMP. Adenylyl cyclases are coincidence detectors, meaning that they are only activated by several different signals occurring together – they are modulated by G-proteins, forskolin, Ca2+/calmodulin, and other class-specific substrates.
Phospholipases are enzymes that hydrolyze specific ester bonds in phosphoglycerides or glycerophosphatidates, converting the phospholipids into fatty acids and other lipophilic substances. Phospholipases are involved in signaling cascades. Phospholipase A1 hydrolyzes the acyl group attached to the 1-position, while phospholipase A2 hydrolyzes the acyl group attached to the 2-position to form fatty acid and lysophospholipid products. Phospholipase A2 is responsible for the release of arachidonic acid from membranes (flow diagram PLA2 pathway). Arachidonic acid is a signalling molecule and is the precursor for eicosanoid signaling molecules, which include leukotrienes and prostaglandins. Some eicosanoids are synthesized from diacylglycerol, and are released from the lipid bilayer by phospholipase C.
Members of the five subtypes of phosphodiesterase enzymes degrade the cyclic nucleotide, second messengers, cAMP and cGDP by hydrolyzing phosphodiester bonds, so they are important in regulation of signal transduction. Phospodiesterase inhibitors, such as caffeine, aminophylline, theophylline and Viagra, prolong or amplify the physiological processes that are mediated by cAMP or cGDP. The phospodiester bond form the linkage between 3'-C of nucleotides and 5'-C of pentose sugars in the backbones of DNA & RNA, rendering phosphodiester bonds particularly important. 3'-phospodiesterase is important in repair of oxidative DNA damage.
Tables Phosphate-handling enzymes Cell signaling RTKs Second Messengers .
Examples of use of phosphorylation/dephosphorylation:
1. signaling networks and signal transduction (two-component system)
2. control of cell cycle
3. regulation of amino acids within Ser > Thr >>>>> Tyr
5. bacterial phosphorylation of the amino acids His and Asp in two-component signaling
6. p53 tumor suppressor gene
7. control of chemotaxis in prokaryotes (two-component system)
Examples of phosphorylation reaction
1. ADP + Pi →ATP synthase→ ATP : during oxidative phosporylation, substrate-level phosphorylation during glycolysis, and PSII light-reactions during noncyclic photophosphorylation (diagram Z-scheme)
2. NADH + Pi→NADPH in catabolic reactions such as photophosphorylation
Examples of dephosphorylation reaction
1. ATP - Pi → ADP in anabolic reactions
2. NADPH -Pi →NADH in anabolic reactions
Examples of signaling phosphorylation/dephosphorylation enzymes: two-component systems :
1. receptor tyrosine kinases (RTKs)
2. protein tyrosine phosphatases (PTPs) - transmembrane and intracellular - (CD45),
3. serine/threonine kinases (activins, inhibins, bone morphogenetic proteins (BMPs), MAP kinases, PKCs, TGF-beta receptors),
4. cAMP dependent protein kinase (Phospholipases (PLD, PLA2), Phosphatidylinositol-3-Kinase,
6. Phosphatases.
The spatio-temporal activation of protein kinases and phosphatases is an important factor in controlling where and when phosphorylation events occur. Anchoring proteins provide a molecular framework that orients these enzymes towards selected substrates. A-kinase anchoring proteins (AKAPs) are signal-organizing molecules that compartmentalize the cAMP dependent protein kinase, phosphodiesterases, and a variety of enzymes that are regulated by second messengers[s].
Phospholipases and phospholipids participate in transmission of ligand-receptor induced signals from the plasma membrane to intracellular proteins, primarily PKC, which is maximally active in the presence of calcium ion and diacylglycerol. PKC activity is mediated by receptors that are coupled to activation of phospholipase C-gamma (PLC-gamma), which contains SH2 domains that enable it to interact with tyrosine phosphorylated RTKs. PI-3K is tyrosine phosphorylated and activated by various RTKs and receptor-associated PTKs. PI-3K is activated by the PDGF, EGF, insulin, IGF-1, HGF and NGF receptors. The p85 subunit of PI-3K is activated by tyrosine phosphorylation, but only the 110 kDa subunit is enzymatically active.
Phospholipases D and A2 (PLD, PLA2) sustain the activation of PKC through their hydrolysis of membrane phosphatidylcholine (PC). Activation of PLC-gamma results in hydrolysis of membrane phosphatidylinositol bisphosphate (PIP2), which leads to an elevation of intracellular second messengers, diacylglycerol (DAG) and inositol trisphosphate (IP3), which interact with intracellular membrane receptors to effect release of stored calcium ions (PKC is maximally active in the presence of calcium ion and diacylglycerol).
Chemotaxis • hormones • neurotransmission • Nitric Oxide • neuronal interconnections • phosphotransfer-mediated signaling pathways • Protein Kinase Signaling Networks • protein tyrosine kinases, PTKs • receptor tyrosine kinases • Receptor Tyrosine Kinases (RTKs) • serine/threonine kinases • signaling gradients • signal transduction • two-component systems • animation MAPK signal transduction :
Signaling pathways:
Pathways ABC transporters : Phosphotransferase system (PTS) : Two-component system : MAPK signaling pathway : Wnt signaling pathway : Notch signaling pathway : Hedgehog signaling pathway : TGF-beta signaling pathway : VEGF signaling pathway : Jak-STAT signaling pathway : Calcium signaling pathway : Phosphatidylinositol signaling system : mTOR signaling pathway : Neuroactive ligand-receptor interaction : Cytokine-cytokine receptor interaction : ECM-receptor interaction : Cell adhesion molecules (CAMs) : Orthologies Transporters (+diseases) : Two-component system : Receptors and channels (+diseases) : Cytokines : Cell adhesion molecules (CAMs) : CAM ligands : CD molecules : GTP-binding proteins :








































0 Comments:
Post a Comment
<< Home