eicosanoid biosynthesis
Eicosanoids are not stored within cells, rather they are synthesized as required in response to hormonal signals. Eicosanoids function close to the site of synthesis (autocrine, paracrine), where they are rapidly deactivated before they enter circulation as inactive metabolites.
Table Eicosanoid Actions
 The first step in eicosanoid biosynthesis is phospholipase-catalyzed release from phospholipids (A2) or diacylglycerol (C) of a 20-carbon essential fatty acid (EFA) containing three, four, or five double bonds (ω-6 DGLA, ω-6 AA or ω-3 EPA, respectively).
The first step in eicosanoid biosynthesis is phospholipase-catalyzed release from phospholipids (A2) or diacylglycerol (C) of a 20-carbon essential fatty acid (EFA) containing three, four, or five double bonds (ω-6 DGLA, ω-6 AA or ω-3 EPA, respectively).
------------------free free fatty acid released from membrane phospholipid
--------------------------↓------------------------↓------------ - ---↓
------------------cyclooxygenase------------epoxidase-------lipoxygenease
------------------- ------↓------------------------↓------------------↓
-------------------- prostanoids----------------EETs-------5-HPETE, HPETEs
----------------.---------↓-------------------------------------------↓
-----------prostaglandins, thromboxanes---------leukotrienes, 5-HETE, HETEs
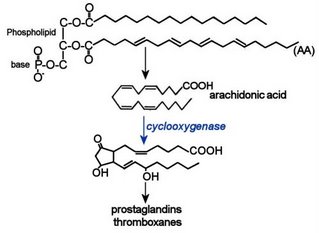 Cyclooxygenase pathways:
Cyclooxygenase pathways:
En route to the prostanoids, membrane-released fatty acids are acted upon by one of two related enzymes, cyclooxygenase-1 (COX-1) or cyclooxygenase-2 (COX-2). Prostanoids include prostaglandins, prostacyclins, and thromboxanes. The cyclooxygenases are alternatively termed prostaglandin endoperoxide H synthases-1 and -2 (PGHS-1, PGHS-2). The COX enzymes are targetted by NSAIDs (non-steroidal anti-inflammatory drugs). ASA and early NSAIDs inhibit both COX-1 and COX-2, and are associated with gastric irritation. The COX-2 inhibitors are more selective and gastro-protective, but inhibition of cardio-protective, anti-coagulative PGF2 is associated with increased risk of cardiovascular thrombotic events.
Both COX-1 and COX-2 catalyze equivalent reactions at different sites. Prostaglandin PGH2 synthase contains two catalytic centers, a cyclooxygenase and a peroxidase. In the enzymatic reactions, two molecules of oxygen are added to arachidonic acid to form a bicyclic endoperoxide then a further hydroperoxy group is added to position 15 to form prostaglandin PGG2. The hydroperoxide is next reduced by a functionally coupled peroxidase reaction to generate the unstable intermediate prostaglandin PGH2. All other prostanoids are derived from the unstable PGH2 intermediate by a variety of different enzymic reactions. Cell type determines the nature and proportions of the various enzymes, which differ in amino acid sequence, structure and cofactor requirements, and so of the prostanoids generated. (more detail, diagram)
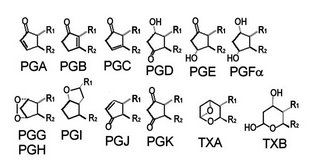
For example:
prostaglandin A synthase : PGH2 → PGA2
prostaglandin D synthase : PGH2 → PGD2
prostaglandin E synthase : PGH2 → PGE2
prostacyclin synthase : PGH2 → PGI2 (prostacyclin)
thromboxane A synthase : PGH2 → TXA2 (thromboxane A)
One prostaglandin can be converted to another by spontaneous rearrangement, dehydration, or isomerization. Thromboxane A is deactivated by non-enzymatic hydrolysis to TXB Lipoxygenases are a family non-heme iron enzymes that catalyze the substitution of oxygen for hydrogen in the bis-allylic position of fatty acids to generate hydroperoxide products, which are further metabolized to leukotrienes and lipoxins. (left -click to enlarge - 5-HPETE is 5S-hydroperoxy-6t,8c,11c,14c-eicosatetraenoic acid, and is generated in the reaction catalyzed by 5-LOX).
Lipoxygenases are a family non-heme iron enzymes that catalyze the substitution of oxygen for hydrogen in the bis-allylic position of fatty acids to generate hydroperoxide products, which are further metabolized to leukotrienes and lipoxins. (left -click to enlarge - 5-HPETE is 5S-hydroperoxy-6t,8c,11c,14c-eicosatetraenoic acid, and is generated in the reaction catalyzed by 5-LOX).
5-LOX employs nuclear-membrane protein cofactor 5-lipoxygenase-activating protein (FLAP). 5-LOX produces the primary precursor 5-HPETE then leukotriene A4 (LTA4), which may be converted into LTB4 (image at left) by the enzyme leukotriene A4 epoxide hydrolase. In animal tissues, lipoxygenase catalyzed reactions with free arachidonic acid produce specific eicosanoid hydroperoxides for 5-LOX, 8-LOX, 12-LOX, and 15-LOX. Lipoxygenases can also generate hydroperoxides from phospholipids within membranes, disturbing the membrane structure.
 In eosinophils, mast cells, and alveolar macrophages, the enzyme leukotriene C4 synthase is employed to conjugate LTA4 plus glutathione to generate leukotriene C4 (LTC4). Glutathione contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side chain. Once LTC4 has been secreted, a glutamic acid moiety is removed from it to produce leukotriene D4 (LTD4), which is cleaved by dipeptidases to generate leukotriene E4 (LTE4). LTC4, LTD4 and LTE4 are termed cysteinyl leukotrienes because all contain cysteine.
In eosinophils, mast cells, and alveolar macrophages, the enzyme leukotriene C4 synthase is employed to conjugate LTA4 plus glutathione to generate leukotriene C4 (LTC4). Glutathione contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side chain. Once LTC4 has been secreted, a glutamic acid moiety is removed from it to produce leukotriene D4 (LTD4), which is cleaved by dipeptidases to generate leukotriene E4 (LTE4). LTC4, LTD4 and LTE4 are termed cysteinyl leukotrienes because all contain cysteine. Epoxytrienoic acids (EETs) are generated from arachidonic acid through the epoxidase (epoxygenase) pathway. (right) This mechanism yields four cis-epoxyeicosatrienoic acids (14,15-, 11,12-, 8,9-, and 5,6-EETs). There are several isozymes of the cytochrome P450 epoxygenase that act upon un-esterified substrates, producing all four EET regioisomers, which may be subsequently esterified. Epoxyeicosatrienoic acids (EETs) are considered antihypertensive because they elicit vasodilation and oppose the K(+)-channel stimulatory actions of 20-HETE, in addition to modulation of the activity of angiotensin II. It has also been proposed that EETs are endothelium-derived hyperpolarizing factors (EDHFs) that mediate the nitric oxide (NO)- and prostaglandin-independent vascular effects of acetylcholine (Ach) and bradykinin.
Epoxytrienoic acids (EETs) are generated from arachidonic acid through the epoxidase (epoxygenase) pathway. (right) This mechanism yields four cis-epoxyeicosatrienoic acids (14,15-, 11,12-, 8,9-, and 5,6-EETs). There are several isozymes of the cytochrome P450 epoxygenase that act upon un-esterified substrates, producing all four EET regioisomers, which may be subsequently esterified. Epoxyeicosatrienoic acids (EETs) are considered antihypertensive because they elicit vasodilation and oppose the K(+)-channel stimulatory actions of 20-HETE, in addition to modulation of the activity of angiotensin II. It has also been proposed that EETs are endothelium-derived hyperpolarizing factors (EDHFs) that mediate the nitric oxide (NO)- and prostaglandin-independent vascular effects of acetylcholine (Ach) and bradykinin.
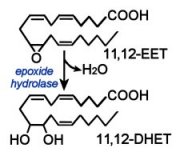 Epoxide hydrolases metabolize EETs to the corresponding dihydroxyeicosatrienoic acids (DHET). Isozymes of the epoxide hydrolases are found in different cellular locations – cytosolic or membrane-bound.
Epoxide hydrolases metabolize EETs to the corresponding dihydroxyeicosatrienoic acids (DHET). Isozymes of the epoxide hydrolases are found in different cellular locations – cytosolic or membrane-bound.
tags [Biochemistry] [Molecular Biology] [eicosanoid] [phospholipid][NSAID]








































0 Comments:
Post a Comment
<< Home